
How mouse retinas and computational biology might help central nervous system injuries
Dr. Jiarui Ding, UBC computer science assistant professor and interdisciplinary researcher, is co-first author of a paper published in Nature Immunology, with a group of 12 co-authors in total.
Some of the researchers began working together when Dr. Ding was a Postdoc at the Broad Institute of MIT & Harvard.
Paper: “Temporal single-cell atlas of non-neuronal retinal cells reveals dynamic, coordinated multicellular responses to central nervous system injury,” Benhar, I., Ding, J., et al. Nature Immunology (2023).
Dr. Ding explains the research, “Generally, in adult mammals such as humans, the central nervous system loses the ability to regenerate compared to many other species. Thus, we need to find ways to protect neurons from dying in diseases, as in many neurodegeneration diseases such as Parkinson's disease, Alzheimer's disease, and so on. Ultimately, we hope to figure out why adult humans lose that regeneration ability, and find ways to protect those neurons from dying.”
The team used mouse retinas in their research because they are established ‘model systems’ with a delicate structure and consisting of many cell types working together to help keep the retina in a healthy, stable condition. If they injured one kind of neuron of the retina (called Retina Ganglion Cells or RGC), the majority of these neurons died in two weeks. But others did survive the injury. “In this study, we wanted to figure out how different cell types of the retina performed during this two-week time period after injuring the retina ganglion cells,” said Dr. Ding. “And ultimately, gain a better understanding of why neurons respond to injury differently.”
An overview of the research
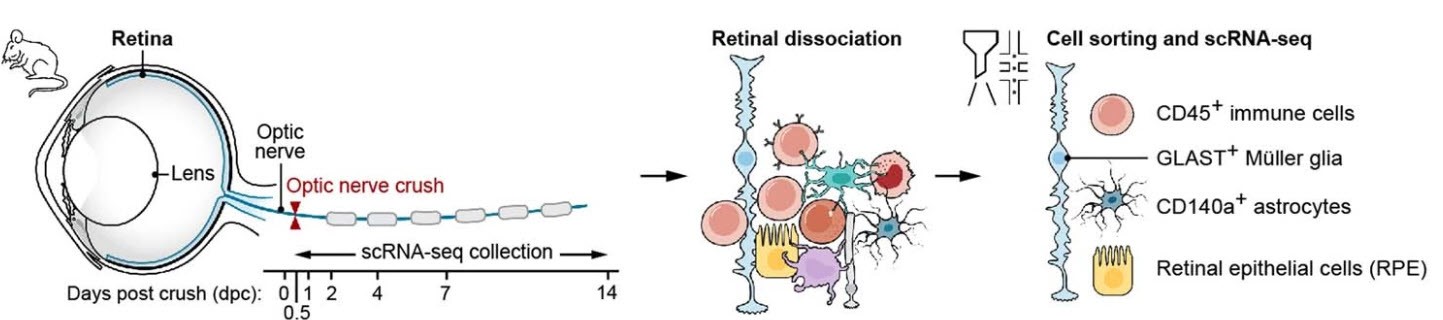
Their research led to a major conclusion: namely, that by creating an atlas of the retina’s non-neuronal cells, they were able to identify roughly three phases of neuronal degeneration: early after injury before retina gangling cell death, an intermediate phase with the death of most retina gangling cells, and late inflammatory resolution equilibrium phase. In the first phase, activation of resident glia involves chemokine signals for leukocyte infiltration. During phase two, concurrent with the peak rate of RGC death, infiltrating monocytes differentiate into distinct macrophage subsets, including Ms4a7+MHC-IIhi, Ms4a7+MHC-IIlo and Gpnmb+ macrophages. Also during this phase, a synchronous interferon response program is induced in astrocytes, Müller glia and microglia. The latter also express a disease-associated microglia signature, which overlaps with that of Gpnmb+ macrophages. Finally, at phase three, glial cells begin to return to their baseline proportions, with enriched interactions among them including TGFβ signaling, indicating restoration of homeostasis.
A short medical dictionary
Leukocytes are colourless cells that circulate in the blood and body fluids and are involved in counteracting foreign substances and disease
Monocytes are a type of white blood cell in your immune system. Monocytes turn into macrophage or dendritic cells when an invading germ or bacteria enters your body.
Glia are non-neuronal cells (i.e. not nerves) of the brain and nervous system. There are a variety of subtypes of glial cells, including astrocytes, oligodendrocytes, and microglia, each of which is specialised for a particular function.
Macrophages are a type of white blood cell that surrounds and kills microorganisms, removes dead cells, and stimulates the action of other immune system cells.
Broad applications
Many of these features also occur in other central nervous system (CNS) pathologies, making the research a broadly applicable resource for people who study central nervous system injury, neuronal degeneration, neuronal degeneration diseases or those who conduct CNS repairs.
“I also think our data could be quite useful for computational algorithm developers. There are not many studies doing time-course single-cell study of diseases,” Ding said.
Ding explained his contribution, “As a computational biologist, I contribute to the design and conduct computational analysis of data, especially single-cell sequencing data, and work with collaborators to help make sense of the data. Scientific discovery is hard because we don’t have pipelines to automatically distill bio-medical knowledge and humans still need to play a big role. But we are developing computational tools to make this process more automatic and efficient.”
Jiarui said the team is thrilled to be published in the high-ranking Nature Immunology, because they believe their work could be of interest to the journal’s wide audience, and help immunology, neuroimmunology, single-cell geonomics, computational biology, and more.
More about Dr. Jiarui Ding